Water is a Remarkable Substance: Yes, plain, ordinary, everyday liquid water is by far the most unique chemical substance in our physical universe. Most of us realize its essential and nourishing qualities, for without water life would not have arisen on our planet. But, water is both life-supporting and immensely destructive at times. That is one of the main reasons to understand its extraordinary properties. To begin with, water (H2O) is a small molecule, with a molecular weight of only 18. By comparison to other similar molecules, it should be a gaseous substance at room temperature, but it is not. For instance, let’s compare water’s physical properties with ammonia (NH3; molecular weight 17), since their molecular weights are almost the same. Well, at room temperature, water is a liquid, with a boiling point of 100oC (212oF), while ammonia is a gas with a boiling point of – 33oC (– 28oF).
Why is this so? Here’s one way to understand it. The oxygen atom in the water molecule (H-O-H) bonds with its two hydrogen atoms in a tight, chummy manner. These strong links are called covalent bonds (O–H bond energy = 463 kJ/mol). In addition, the oxygen atom in each water molecule can also bind lightly to two other neighboring hydrogen atoms. These partial electrostatic links are called hydrogen bonds (O…H bond energy = 21 kJ/mol). As we shall see, these hydrogen bonds are, in fact, the magical ingredient that gives water its special properties.
Using space filling models, we can visualize these strong and weak interactions between oxygen and hydrogen atoms in water molecule as follows:
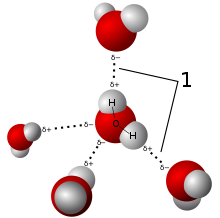
As seen above, liquid water has an almost tetrahedral crystalline structure, yet exists in a fairly loose and flexible state, with a network of hydrogen-bonded molecules at room temperature. Whereas, poor ammonia molecule, with its one nitrogen atom, each covalently bonded to three hydrogen atoms (N–H bond energy = 391 kJ/mol), can only bind lightly to one neighboring hydrogen atom (N…H bond energy = 13 kJ/mol), leaving two hydrogen atoms without additional bonding partners. Since ammonia does not have water’s more compact, loosely interactive network of hydrogen-bonded molecules, it is in a gaseous state at room temperature.
So, what has all this to do with climate change and global warming?
Quite a bit, it turns out. It has to do with knowing where all the human-induced global warming’s heat content is being stored on the surface of our planet. We measure the capacity to store heat of any substance by determining its specific heat. Because of liquid water’s intricate network of hydrogen bonds, allowing it to absorb a great amount of heat for each degree of temperature rise, the specific heat of seawater (3.85 kJ/kg oC) is well over three times greater than the specific heat of air (1.16 kJ/kg oC).
Taking into account Earth’s oceanic mass (5.95 x 1018 kg) and its atmospheric mass (5.14 x 1018 kg), and factoring in the specific heats of seawater and air, the estimated heat capacity of ocean is nearly 900 times greater than the atmosphere (5.3 x 1024 J/oC versus 5.95 x 1021 J/oC). This leads to the dramatic difference in global warming’s heat distribution between Earth’s ocean and its atmosphere. This is displayed below:

This indicates that an overwhelmingly large fraction – greater than 90% – of human-induced global warming’s heat content resides in the oceans. That is, a 0.5oC to 1oC increase in average global temperature over pre-industrial period is like a potential “mega-time bomb”, which can release its immense reservoir of additional oceanic heat content into a series of highly destructive cyclonic activities in different regions of the globe. The net result is greater frequency of high intensity hurricanes, typhoons and superstorms at present and in the foreseeable future.
Water Vapor as a Greenhouse Gas: Before going any further, we must clear up one of the most puzzling aspects of climate change. Yes, water in its vapor state is a greenhouse gas! In fact, it is the most abundant greenhouse gas in the atmosphere, absorbing Earth’s outgoing infra-red radiation from escaping to space. Thus, without water’s major contribution to Earth’s greenhouse effect, our planet would not be habitable. But, water as a greenhouse gas is not part of human-induced global warming; it has always been a part of our planet’s baseline atmospheric warming phenomena. Here again, water’s unique property of being in its liquid state at room temperature plays a role. For when water evaporates from Earth’s surface, it condenses back to its liquid state quite readily in the form of precipitation – rain, hail and snow. It is part and parcel of Earth’s vast hydrological cycle. In other words, water vapor has a saturation point in the atmosphere after which no more water molecules can remain in its gaseous state.
Now, unlike water, carbon dioxide (CO2) and methane (CH4), are in their gaseous state at room temperature and do not condense back to Earth’s surface. This is most unfortunate. For once they are emitted into the atmosphere, they remain in the air for decades or even centuries. Thus, beyond a certain beneficial amount they contribute to Earth’s background greenhouse effect, they become a major liability to the survival of the majority of our planet’s life-forms, including humans. Let’s take a look at carbon dioxide’s atmospheric concentrations as observed in Antarctic ice core measurements for the past 800,000 years:

As is readily apparent, not until the early 1900s, did the atmospheric concentration of carbon dioxide exceed 300 parts per million (ppm). Today, CO2 concentration levels in the atmosphere has crossed its 400 ppm mark and continues steadily increasing every year. Lest we forget, there is one major source for this unprecedented increase of atmospheric CO2 concentration – our society’s industrial, commercial and residential activities that are dependent on the combustion of fossil fuels – oil, natural gas and coal – as our main energy source.
Atmosphere-Ocean Interaction: In examining this intricate physical phenomenon, let’s try to understand why oceanic cyclonic activity occurs in the first place. If the Earth were a non-rotating globe, there would be no such cyclonic activity. In that case, only a longitudinal circulation of atmospheric wind would occur between the warmer equatorial region (high pressure zone) to the cooler Northern and Southern Polar Regions (low pressure zones). Because of Earth’s rotation on its axis, large-scale atmospheric wind currents moves in clock-wise directions in the Northern Atlantic and Pacific Oceans, and in the counter clock-wise directions in the Southern Hemisphere, including the Indian Ocean. This is caused by the Coriolis Effect – apparent inertial forces formed between rotating and non-rotating bodies – acting on the Earth’s surface, impacting both atmospheric and oceanic currents.
The dynamics of hurricane formation is fairly complex, but stripped to its essentials it can summed up thus: (a) they are formed in warm tropical oceanic regions near the Equator and move generally in the Northwest direction in the North Atlantic Ocean; (b) most of their physical energy is drawn from the rising evaporation of warm oceanic water, causing an area of low pressure at the ocean’s surface below; (c) this region of low pressure causes the cooler air in the upper atmosphere to move downward; (d) this, in turn, forces the warm, moist air on the ocean’s surface to spiral upwards in a counter-clockwise direction – again due to a “local” Coriolis Effect, even though the overall cyclonic movement in the Northern Hemisphere is clockwise; and (e) if these atmosphere-ocean interactions are further sustained, tropical storms and hurricanes of various wind velocities and energy intensities are formed.

In addition to high intensity hurricanes and typhoons, drenching rainfall events followed by severe flooding can occur from abnormally heavy moisture-laden storm clouds. This is what seems to have happened lately. With the public’s attention fixed on Hurricane Harvey’s disastrous impact on Houston, few of us noticed that in late August more than 1,200 people died from monsoon-related floods in India, Nepal and Bangladesh, with over 40 million people adversely affected or displaced in the South Asian region. With unremitting rains continuing to fall on Bangladesh, a country of 165 million people, one-third to half the country is currently under water.
Earlier in July, Beijing was inundated by a sudden rainfall, their heaviest in 60 years, which completely paralyzed the metropolitan region. In the same month, flash floods from torrential rains in Istanbul turned major thoroughfares into rivers and streams. Similar heavy rainfall occurred this summer in Northern Ireland and in parts of England and Scotland, while major rainstorm threats have lately been sounded for several regions of Pakistan and Zimbabwe. It appears that brutally punishing rainstorms and widespread flooding have now become the new normal on a worldwide scale.
Global Ocean-Land Temperature Trends: Now let’s revisit the amount of heat stored in the ocean from 1960 to the present time. The following figure shows that the bulk of changes in total heat content changes are found in the upper 700 meters of the ocean surface, though a significant amount is still present in the 700 to 2,000 meters range, while the combined energy stored in land, ice and atmosphere is less than 10% of the Earth’s total heat content changes.

Thus, any global warming trend analysis that does not include ocean’s surface temperature, such as exclusively relying on satellite recording of Earth’s atmospheric temperature trends would be extremely misleading. According to the National Atmospheric and Oceanic Administration’s (NOAA) latest Global Climate Report (July 2017), based on global ocean-land temperature measurements, the eight warmest years so far recorded in decreasing order of severity were: 2016, 2015, 2014, 2010, 2013, 2005, 2009, 1998. By mid-year, 2017 appears to be the 2nd or 3rd warmest ever recorded. https://www.ncdc.noaa.gov/sotc/global/201707
Hurricane Harvey, Metropolitan Houston and Local Policy Makers:

Much has been written about the devastating impact Hurricane Harvey has had on Houston and its outlying suburbs when the storm’s moisture-laden and 130 miles per hour winds made initial landfall on August 24 this year. As much as the higher than normal temperatures this summer in the Gulf of Mexico contributed to increasing the severity of this unprecedented category-4 hurricane and its ensuing once-in-a 1,000 year flood from over 50 inches of rainfall, the lackadaisical attitude and willful indifference to flood control and storm-preparedness on the part of many local and state officials prior to this combined natural/human-induced disaster was even more shocking and reprehensible.
In an in-depth article, “Boomtown, Flood Town”, jointly written by the staff of ProPublica and The Texas Tribune, and published last year (December 7, 2016;), they offer a pretty unflattering picture of what the rest of country recently became well aware of:
Climate change will bring more frequent and fierce rainstorms to cities like Houston. But unchecked development remains a priority in the famously un-zoned city, creating short-term economic gains for some while increasing flood risks for everyone. . . . “More people die here than anywhere else from floods,” said Sam Brody, a Texas A&M University at Galveston researcher who specializes in natural hazards mitigation. “More property per capita is lost here. And the problem’s getting worse.” Why? Scientists, other experts and federal officials say Houston’s explosive growth is largely to blame. As millions have flocked to the metropolitan area in recent decades, local officials have largely snubbed stricter building regulations, allowing developers to pave over crucial acres of prairie land that once absorbed huge amounts of rainwater. That has led to an excess of floodwater during storms that chokes the city’s vast bayou network, drainage systems and two huge federally owned reservoirs, endangering many nearby homes. . . Houston’s two top flood control officials say their biggest challenge is not managing rapid growth but retrofitting outdated infrastructure. Current standards that govern how and where developers and residents can build are mostly sufficient, they say. And all the recent monster storms are freak occurrences — not harbingers of global warming or a sign of things to come. (emphases added) (https://projects.propublica.org/houston-cypress/)
The observation that “recent monster storms are freak occurrences – not harbingers of global warming” is the unfortunate view of a lot of climate change deniers and a good number of high-level government officials in this country with their collective heads buried in sand. Since another “freak” storm visited their fair city yet again – three major flooding events in three consecutive years – this question should finally be posed to the presiding officials in Houston: When will you stop ignoring all the signs of human-induced climate change and start worrying about your region’s days of reckoning now so clearly written on the wall?
Postscript: Water, Hydrogen Bonds and Life on Earth: Let’s take a quick look how water molecules and other biological substances, with their truly magical hydrogen bonds, play a role in creating and sustaining life on our planet. There is absolutely no chance any animal, plant or microbial life-form could exist without water. Liquid water, with its vast interactive network of hydrogen bonds, constitutes the largest amount of the animal body’s mass – from 50 to 95%, depending on the organ or part of the body’s structural features. Without the presence of water, no metabolic reaction, cellular interaction or myriads of other biochemical activities could take place.
Two of the most important biochemical substances in every life-form – protein and DNA molecules – hydrogen bonds play a crucial role in their structural make-up. In protein molecules, including the tirelessly active and enormously efficient enzymes that catalyze metabolic reactions, hydrogen bonds provide internal stability to the macromolecule. This is accomplished by forming alpha-helical secondary coils and adjacent pleats among neighboring amino acid residues in the protein’s chain-like primary structure.
Similarly, DNA molecules, which provides the cell’s all-important genetic code, has a double-helical structure, with its four nucleotide bases (adenine, A, thymine, T, guanine, G, cytosine, C) aligning themselves pair-wise by the assistance of hydrogen bonds: A…T and G…C. Its firm, yet lightly-held hydrogen bonds between these nucleotide bases, allows the DNA molecule to smoothly uncoil and recoil during its transcription phase in the cell’s nuclease.
In effect, we have discovered one of nature’s hitherto well-kept secrets: how ubiquitous hydrogen bonding links provide the underlying structural edifice of water, protein and DNA molecules in all living organisms. In sum, without hydrogen bonds, there would be no life!
